Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a primary disease of heart muscle that results in fibrofatty replacement of the right ventricle, and the subepicardial region of the left ventricle. Patients are at high risk of ventricular tachyarrhythmias and sudden death (see the images below). The prevalence of ARVC has been estimated at 1:2500 to 1:5000. In series of sudden cardiac death, this condition represents the cause of 2-5% of deaths in young adults, with higher incidences in exertional deaths1 and in certain regions of Europe.2 ARVC is a relatively new entity and its incidence worldwide is not yet established. In addition to right ventricular involvement, the left ventricle is also involved in a significant percentage of cases. The etiology of arrhythmogenic right ventricular cardiomyopathy (ARVC) is unknown. The presence of inflammatory infiltrates in most cases seen at autopsy has led to the theory that it is a resolving myocarditis.3 Families have been described with phenotypic alterations ranging from myocarditis to fibrofatty cardiac infiltrates, and imaging studies have demonstrated a progression from myocarditis to ARVC. More recently, genetic studies based on animal models, rare families with coincidental skin lesions, and, family studies of isolated cardiomyopathy have led to the discovery of mutations in genes related to the desmosome. ARVC may possibly be an inflammatory process that is modulated by genetic influences in desmosome-related proteins. Although initially described in the right ventricle, microscopic examination of the heart in autopsy cases of arrhythmogenic right ventricular cardiomyopathy (ARVC),4 or with sensitive imaging techniques,5 has established that most patients with ARVC have biventricular involvement. The left ventricular involvement is typically subepicardial, the changes are more of fibrosis than fat or fibrofatty infiltration, though at times the change is probably similar to that seen in the right ventricle. However, because the right ventricle is relatively thin, the subepicardial nature of disease is difficult to appreciate in imaging studies and has only infrequently been investigated at autopsy.6 Atrial involvement appears not to have been adequately investigated. The primary symptoms of arrhythmogenic right ventricular cardiomyopathy (ARVC) are related to arrhythmias and conduction disturbances.7 However, this "disease" is frequently symptomless, with the first manifestation being sudden unexpected death.1 The pathologist is likely to encounter the disease at autopsy in cases of sudden death.2 Approximately three quarters of sudden deaths are exertional. ARVC is generally limited to the young, especially males younger than 40 years, although the disease has been described up to the ninth decade.8 This condition is extremely uncommon under the age of 10 years. In cases of arrhythmogenic right ventricular cardiomyopathy (ARVC), the typical findings at autopsy include areas of thinning of the right ventricle, with fibrofatty change, in areas where fat is not normally found (outflow region, posterior wall) (see the following image). Pathologic findings at autopsy are wide ranging, depending on the stage and extent of the disease. Histologically, the ventricle in arrhythmogenic right ventricular cardiomyopathy (ARVC) may show a range of changes, with the dominant feature being fatty or fibro fatty replacement of the myocardium (see the image below). It may appear randomly "moth-eaten," with destruction of the normal myocytes via inflammation, scarring, and fat replacement; show patchy areas of full thickness loss of myocytes and replacement by fibrofatty tissues; or show inflammation composed of lymphocytes and macrophages, with only rare myocyte necrosis. The myocytes in the involved areas appear attenuated, with loss of cross-striations and areas of myofibrillar loss imparting a "bubbly" appearance. The changes are nonspecific, as fat may be a component of ischemic scarring and myocyte vacuolization may be seen in other forms of cardiomyopathy, as well as inflammatory infiltrates. In cases of unexplained exertional death in young adults, submitting multiple sections of ventricular septum and right ventricle to exclude arrhythmogenic forms of cardiomyopathy is reasonable, as ARVC most typically involves the right ventricle and may be difficult to see grossly; additionally, hypertrophic cardiomyopathy may involve the interventricular septum without obvious grossly abnormalities. Because both of these entities often cause exertional death, taking multiple sections of right ventricle and ventricular septum in cases of exertional deaths is prudent. In general, immunohistochemistry (IHC) is not useful in the diagnosis arrhythmogenic right ventricular cardiomyopathy (ARVC). Some investigators have postulated that diffuse loss of desmosomal proteins, as seen immunohistochemically, is suggestive of the diagnosis of ARVC. These proteins include connexin-43 and plakoglobin.12 Family studies have suggested that arrhythmogenic right ventricular cardiomyopathy (ARVC) is caused by genetic alterations in desmosomal proteins, especially plakoglobin and desmoplakin.13,14,15,16,17 A family study demonstrated desmosomal mutations in just fewer than 20% of patients.5 Because autopsy studies are skewed, to fatal arrhythmogenic right ventricular cardiomyopathy (ARVC) and byconsent for autopsy, it has been assumed that ARVC is uniformly lethal. The clinical diagnosis has been hampered by the nonspecificity of ECG and imaging findings. With improvements in cardiac MRI and computed tomography scanning, the clinical diagnosis has become more precise.Arrhythmogenic Right Ventricular Cardiomyopathy
Definition
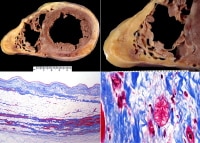
Arrhythmogenic right ventricular cardiomyopathy (ARVC), biventricular, autopsy heart in a young man who died suddenly playing basketball. Top left demonstrates increased fat in the outer walls of the right ventricle and left ventricular posterolateral walls. A higher magnification of the right ventricle is seen at the top right image; the anterior wall is nearly completely replaced with fat, and fibrofatty irregular posterior wall involvement is seen. Note that no actual thinning of the wall itself exists, although the muscular portion is in some areas completely missing.
The bottom left image demonstrates a full thickness of the right ventricle stained with Masson trichrome. The residual muscle is present only in a band-like area of scarring, and subepicardial scarring is present as well. The characteristic myocyte vacuolization, depicted in the bottom right image, is seen in nearly all areas of ARVC within the scarred areas.Epidemiology
Etiology
Location
Clinical Features and Imaging
Several clinical criteria for diagnosis have been proposed, including those of Working Groups on Myocardial and Pericardial Disease and Arrhythmias of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the World Heart Federation in 2000,9 and a revision in 2002.10 Symptoms, hemodynamics, and electrocardiographic (ECG) findings are relatively nonspecific, but advances in cardiac imaging, especially magnetic resonance imaging (MRI), have increased the reproducibility of clinical diagnosis.Gross Findings
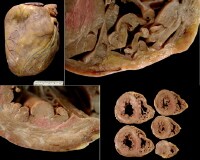
Gross findings at autopsy, ventricular arrhythmogenic right ventricular cardiomyopathy (ARVC). A suspicion of the diagnosis occurs if the right ventricle appears dilated, especially fatty, upon initial inspection of the heart (top left). Actually, aneurysms occur in approximately 35% of patients when evaluated by imaging.
At autopsy, dilatation such as seen in this example occurs in 40-50% of cases, with true aneurysm being less common. The fibrofatty involvement of the right ventricle (upper right) is typically patchy; in this case, a segment of fatty replacement exists, without significant thinning or aneurysm, features that were once considered pathognomonic of the disease. Left ventricular scarring is generally subepicardial, but in this case (bottom left), the scarring is more random, reflecting the phenotypic heterogeneity of ARVC. On cross-section (short axis cuts) seen at low magnification (bottom right), the changes are not particularly striking of a particular cardiomyopathy; it is almost the rule that careful examination for fibrofatty infiltrates is necessary for adequate sampling that will lead to the diagnosis.
Some investigators have postulated that ARVC begins in the right ventricle and progresses to biventricular involvement and heart failure. However, newer imaging techniques reveal that left ventricular involvement is typical from the onset.5 Autopsy studies from patients who die suddenly with the disease demonstrate biventricular involvement in most cases, with subepicardial left ventricular involvement and right ventricular fibrofatty infiltration. Left ventricular involvement grossly is that of scars in the subepicardial region, randomly throughout the ventricle; the fat is difficult to appreciate, and often the fibrosis is more conspicuous (see the image above). The ventricular septum is relatively spared. Aneurysms of the right ventricle, while well described in the literature (triangle of dysplasia), are present in only a minority of patients. In a small proportion of cases, gross abnormalities may in some cases be minimal, with the diagnosis made onlymicroscopically.11Microscopic Findings
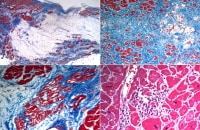
Microscopic features of arrhythmogenic right ventricular cardiomyopathy (ARVC). These images are histologic sections from the heart illustrated in the previous gross images. The top left demonstrates an area of fat in the right ventricle, with fibrous tissue highlighted by the Masson trichrome stain. The areas of fat are irregular, in contrast to the normal "marbling" appearance seen in the anterior right ventricle, in which relatively even, linear fatty areas exist. The fat in ARVC is considered "metaplastic" or secondary to an area of previous myocyte destruction.
In contrast to the right ventricle, the areas of involvement in the left ventricle (top right) often show fat and more fibrous tissue. A higher magnification demonstrates altered myocytes with vacuoles, which are seen in all areas of fibrofatty infiltration (bottom left). Depending on the degree of sampling and definition of myocarditis, inflammation with myocyte necrosis is seen in over 25% of cases (bottom right).
The diagnosis of ARVC is generally made pathologically at autopsy or at examination of explanted hearts at orthotopic heart transplantation. Endomyocardial biopsy is unreliable, as sampling the apical septum does not typically reveal diagnostic material, and biopsies of the septal endomyocardium may not show the characteristic lesions.Immunohistochemistry
Molecular/Genetics
Prognosis and Predictive Factors
In some centers, the incidence of ARVC is highest at the surgical pathology bench where hearts removed (at transplantation) for congestive failure, have easily been mistaken for dilated cardiomyopathy. This may also represent the unfortunate decrease in cases that come to autopsy.
Studies of families with ARVC in recent years have suggested that with antiarrhythmic treatment, including implantation of defibrillators, the prognosis may be improved. Young age, family history of juvenile sudden death, QRS dispersion greater than or equal to 40 ms, T-wave inversion, left ventricular involvement, ventricular tachycardia, syncope, and previous cardiac arrest are the major risk factors for adverse prognosis. Preparticipation screening for sport eligibility has been proven to be effective in detecting asymptomatic patients and sport disqualification has saved lives; sudden death in young athletes has substantially declined.2 Differential Diagnosis
At autopsy, the major differential diagnosis includes normal fat deposits in the right ventricle, and subepicardial scars of ischemic origin. Adipose tissue is normally present in the right ventricle, especially in the anterior wall toward the apex, but no associated fibrosis or myocyte changes exist. In the left ventricle, subepicardial scars can be associated with ischemic heart disease, especially if there is embolization of thromboembolic material from epicardial coronary lesions.Multimedia

Media file 1: Arrhythmogenic right ventricular cardiomyopathy (ARVC), biventricular, autopsy heart in a young man who died suddenly playing basketball. Top left demonstrates increased fat in the outer walls of the right ventricle and left ventricular posterolateral walls. A higher magnification of the right ventricle is seen at the top right image; the anterior wall is nearly completely replaced with fat, and fibrofatty irregular posterior wall involvement is seen. Note that no actual thinning of the wall itself exists, although the muscular portion is in some areas completely missing.
The bottom left image demonstrates a full thickness of the right ventricle stained with Masson trichrome. The residual muscle is present only in a band-like area of scarring, and subepicardial scarring is present as well. The characteristic myocyte vacuolization, depicted in the bottom right image, is seen in nearly all areas of ARVC within the scarred areas.
Media file 2: Gross findings at autopsy, ventricular arrhythmogenic right ventricular cardiomyopathy (ARVC). A suspicion of the diagnosis occurs if the right ventricle appears dilated, especially fatty, upon initial inspection of the heart (top left). Actually, aneurysms occur in approximately 35% of patients when evaluated by imaging.
At autopsy, dilatation such as seen in this example occurs in 40-50% of cases, with true aneurysm being less common. The fibrofatty involvement of the right ventricle (upper right) is typically patchy; in this case, a segment of fatty replacement exists, without significant thinning or aneurysm, features that were once considered pathognomonic of the disease. Left ventricular scarring is generally subepicardial, but in this case (bottom left), the scarring is more random, reflecting the phenotypic heterogeneity of ARVC. On cross-section (short axis cuts) seen at low magnification (bottom right), the changes are not particularly striking of a particular cardiomyopathy; it is almost the rule that careful examination for fibrofatty infiltrates is necessary for adequate sampling that will lead to the diagnosis.
Sunday, October 17, 2010
(Enlarge Image)
(Enlarge Image)
Subscribe to:
Post Comments (Atom)
0 comments:
Post a Comment