The World Health Organization (WHO) defines a cardiac myxoma as a neoplasm composed of stellate to plump cytologically bland mesenchymal cells set in a myxoid stroma.1 Epidemiological characteristics of cardiac myxomas are best divided into the following 2 categories: those that arise sporadically (95%) and those that occur in association with a so-called “myxoma syndrome” (5%). Familial cardiac myxomas typically arise as part of the Carney complex (see Molecular/Genetics). Cardiac myxomas occurring in this setting arise in combination with 2 or more of the following lesions: skin myxomas, cutaneous lentiginosis, myxoid fibroadenomas of the breast, pituitary adenomas, and large-cell calcifying Sertoli-cell tumors of the testis. Although numerous theories have been posited regarding the etiology of cardiac myxomas, their precise histogenesis remains largely enigmatic. For a time, cardiac myxomas were believed to arise from mural thrombi.6However, the differences between myxomas and thrombi are substantial. Although mural thrombi tend to occur in individuals with underlying heart disease and in many locations within the heart (atrial appendages, atria, ventricles), myxomas arise with astonishing consistency in one location, primarily adjacent to the fossa ovalis. Most myxomas (75%) are located in the left atrium, with the remainder (18%) being found predominately in the right atrium.10 Equal incidence is found between the left and right ventricles (3% each), and only a very small fraction (<1%) style="font-size: 0.85em; line-height: 0; ">11,12,13,14 Cardiac myxomas elicit a wide variety of symptoms in patients, largely dependent on size and location of the tumor. Individuals with cardiac myxomas can present at any point along the clinical continuum, ranging from complete asymptomatology (particularly with tumors less than 40 mm in size) to sudden death, usually owing to acute obstruction or embolization.17,18 Although the clinical spectrum can be rather wide, most affected individuals present with one or more of a triad of symptoms (the so-called "myxoma triad"), which includes embolic phenomena, intracardiac flow obstruction, and constitutional symptoms.19,20,21,22 These symptoms, however, are rather nonspecific and often lead to a misdiagnosis owing to the relative rarity of myxomas (or cardiac tumors in general) that are encountered by most clinicians.23 Open table in new window The vast majority of cardiac myxomas manifest as cavitary gelatinous masses that arise adjacent to the fossa ovalis in the left atrium.19 They are typically pedunculated but can also arise in a sessile fashion, ranging in size from as small as a few millimeters to upwards of 15 cm.28,24 Cardiac myxomas have been reported to weigh as much as 250 g.1 The diagnosis of cardiac myxoma is primarily dependent on the identification of the stellate to plump, cytologically bland mesenchymal cells, so-called “myxoma” or “lepidic” cells described in the WHO definition above. Myxoma cells are frequently stellate with eosinophilic cytoplasm and indistinct cell borders. Their ovoid nuclei are typically pale with open chromatin. Nucleoli may be prominent. Most studies that set forth to define myxomas on an immunohistochemical basis have yielded somewhat conflicting results, making immunohistochemistry somewhat unhelpful in the diagnosis of cardiac myxomas. Myxoma cells exhibit immunoreactivity to calretinin (75-100%), vimentin (>50%), and alpha-1 antichymotrypsin.32,19,33 Approximately 5% of cardiac myxomas are associated with a heritable disorder that also includes spotty pigmentation of the skin and endocrinopathy, collectively referred to as the Carney complex.10 Prior to Carney’s description of this form of multiple endocrine neoplasia, individuals with familial myxoma were described as having either the nevi, atrial myxoma, myxoid neurofibroma, and ephelides (NAME) syndrome35 or the lentigines, atrial myxoma, and blue nevi (LAMB) syndrome.36 Supplemental criteria are as follows: Although the clinical course of cardiac myxomas is considered by most to be entirely benign, isolated cases of cardiac myxomas undergoing so-called “malignant change” have been reported.39,40,41 Reports typically cite areas of hypercellularity, necrosis, and atypia as rationale for this designation; however, the follow-up in many of these instances has been incomplete, leaving little objective evidence of a true malignant process. Additionally, whether these cases represent misdiagnosed myxoid sarcomas, a distinct and separate entity, remains a relevant question. The factor that appears most predictive of a recurrence is if the myxoma has arisen in the setting of a familial myxoma syndrome. Patients with sporadic myxomas have a 1-3% recurrence rate compared with those patients with familial myxomas who experience recurrences 10-20% of the time.46,21 The likelihood of developing a recurrence has been associated with abnormal deoxyribonucleic acid (DNA) ploidy patterns, mostly in these familial myxomas.47 Incomplete excision is thought to be the most likely reason for developing recurrence in sporadic tumors. Cellularity and mitotic index do not seem to consistently correlate with recurrence. Close clinical follow-up is recommended for individuals diagnosed with familial cardiac myxomas.Cardiac Myxoma
Definition
Epidemiology
Sporadic cardiac myxomas have been described in individuals as young as stillborn infants and as old as 97 years.2,3 Although they can be found anywhere in this very wide age range, sporadic cardiac myxomas are clearly more common among adults, who present at an average age of 50 years; furthermore, sporadic cardiac myxomas occur approximately twice as often in women as men.3,4 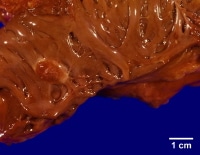
This gross photograph is an excellent example of a sessile myxoma arising within the left atrium. Note how the myxoma has a very broad attachment to the underlying myocardium.
Important demographic differences exist between the sporadic and familial cardiac myxomas. Familial cardiac myxomas appear to occur more commonly in men (2:1) and typically present in the third decade rather than the fifth decade.5Etiology
Furthermore, histologically, myxomas do not organize into fibrous tissue or show stratification, a feature classic of mural thrombi. Cardiac myxomas also behave differently than thrombi in tissue culture studies.7 This body of evidence in support of the neoplastic nature of myxomas has led to the consensus that cardiac myxomas are not of thrombotic origin and are, indeed, neoplastic.8
The impetus for this neoplastic transformation is equally as unclear. Although genetics (see Molecular/Genetics) clearly plays a role in myxoma syndromes, genetics does not appear to offer a consistent explanation in sporadic cases. A recent study centers on the possibility of an infectious etiology. Li and colleagues recently reported finding evidence for HSV-1 infection in 70% of a relatively small cohort (n=17) of surgically resected, sporadic cardiac myxomas.
Regardless of the precise etiology of cardiac myxomas, the morphologic, ultrastructural, and immunoperoxidase studies done to date suggest that the neoplastic cells are of primitive multipotential mesenchymal origin.9,8,7Location
These tumors are typically found arising from the region of the fossa ovalis. For a time, myxomas were thought to arise from minute endocardial endothelial structures known as “Prichard structures,” located primarily in the fossa ovalis.15 This theory has been largely discounted by recent studies that show no relation between the seemingly age-related Prichard structures and myxomas.16
Cardiac myxomas arising as part of the Carney complex occur somewhat less commonly in the left atrium (62%) and are often multicentric, occasionally involving multiple chambers.5 Clinical Features
Symptoms of intracardiac flow obstruction are the most commonly described manifestation, occurring in more than half of individuals with cardiac myxomas.1,24 Typically, these individuals show symptoms of left-sided heart failure (dyspnea, orthopnea, fatigue) or syncope. Likewise, right atrial myxomas can manifest with symptoms of right-sided heart failure (eg, systemic edema, hepatomegaly). Occasionally, a systolic or diastolic murmur can be auscultated. In some instances, the astute clinician may hear a characteristic “tumor plop” in early diastole.24
Somewhere on the order of 30-40% of individuals with cardiac myxomas experience embolic phenomena. Sites of embolization include the central nervous system, kidneys, extremities, and coronary arteries. The clinical manifestations of this embolization are broad and arise dependent upon the tissue downstream of the embolus. Note that paradoxical embolism may occur in individuals with an anatomically patent foramen ovale.25 Myxomas can also uncommonly serve as a nidus for infection.26 When this occurs, symptoms parallel those of infective endocarditis.
Nonspecific constitutional symptoms have been reported in anywhere from 20-60% of individuals with cardiac myxomas.1,10,24 These include such symptoms as fever, arthralgia, myalgia, and weight loss.21 Laboratory findings can include elevated erythrocyte sedimentation rate (33%), normochromic anemia (15%), and thrombocytopenia (5%). These findings are likely due to the tumor’s constitutive ability to elaborate interleukin (IL)-6, a cytokine that induces the acute-phase response. Individuals who experience these constitutional symptoms have been found to have higher serum levels of IL-6, with these levels dropping precipitously following removal of the tumor.27
Differential diagnosisDifferential Diagnosis Differentiating Features Thrombus • Zonation pattern with fibrin
• Absence of myxomas cellsMyxoid sarcoma • Pleomorphic spindle cells
• Lacks siderophagesPapillary fibroelastoma • Located on valvular cusps
• Endothelial-lined avascular papillary frondsCalcified amorphous tumor of the
heart (Cardiac C.A.T.)• Prominent calcification
• Absence of myxoma cellsMesothelial incidental cardiac
excrescence (M.I.C.E.)• Lack myxoid stroma
• Previous history of cardiac procedureFibroma • Abundant collagenous stroma
• Typically ventricularIntracavitary hemangioma • lobular arrangement of vessels
• typically lack myxoid stroma
• vessels surrounded by actin-positive pericytesMetastatic carcinoma • Myxomas rarely contain glandular elements
• Patients typically have a history of carcinomaGross Findings
Cardiac myxomas can have a smooth or papillary surface and may have thrombus adherent. Both the papillary excrescences as well as the surface thrombi can be friable in nature and undergo embolization. On cut section, they often have a variegated appearance, with areas of pearly white, pale-gray, and yellow tissue punctuated by patchy dark-red hemorrhage, gritty necrosis, or calcification.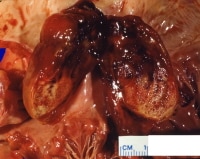
This gross photograph shows a sizable myxoma arising in the left atrium adjacent to the mitral valve. The tumor has been bivalved to demonstrate its variegated appearance. Clearly seen are pearly white, pale-gray, and yellow areas of tissue punctuated by patchy dark-red hemorrhage.
Microscopic Findings
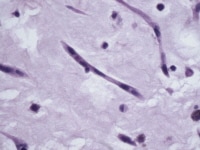
This microscopic image shows a so-called "myxoma cell." Myxoma cells are frequently stellate with eosinophilic cytoplasm and indistinct cell borders. Their ovoid nuclei are typically pale with open chromatin.
Architecturally, myxoma cells form rings, cords, and nests that are often closely associated with capillaries. The cells can also exist singly as stellate cells in a myxoid stroma. This stroma is composed of variable amounts of proteoglycans, collagen, and elastin and often contains lymphocytes, plasma cells, and histiocytes. Extravasated erythrocytes and hemosiderin deposition are invariably present. Thin-walled blood vessels (occasionally large and cavernous) can be seen throughout the lesion, with thick-walled blood vessels typically present near the pedicle or attachment. Myxomas have been shown to elaborate vascular endothelial growth factor (VEGF), likely contributing to the high vascularity frequently encountered in these tumors.29
Secondarily, fibrosis, necrosis, thrombosis, calcification, or gamma Gandy body (calcific/siderotic elastic fiber degeneration) formation can be identified. These secondary, degenerative changes are often attributed to a long clinical course and are more frequently encountered in right atrial myxomas. Knowing that myxomas frequently have adherent thrombus on their surface is an important fact that the surgical pathologist should be familiar with, to avoid a misdiagnosis.
In addition to the more common histopathologic features of cardiac myxomas described above, heterologous elements such as columnar epithelium (sometimes forming glands),10,30 bone formation,31 extramedullary hematopoiesis,10 and thymic rests10 have been described. However, these elements are quite uncommon and have been found in less than 2% of cardiac myxomas.1
No study has been able to show consistently reproducible differences at the gross or microscopic level in sporadic and familial myxomas.Immunohistochemistry
Calretinin, in particular, has been proven quite useful in discriminating cardiac myxomas from mural thrombi and papillary fibroelastomas, with the later entities lacking immunoreactivity. Cardiac myxomas have shown to be variably immunoreactive to S-100, smooth muscle actin, desmin, alpha-1 antitrypsin, synaptophysin, NSE, Factor VII, CD34, and CD31.32,1,19,34 Although myxoma (lepidic) cells are not themselves immunoreactive to cytokeratins, myxomas can exhibit reactivity within heterologous glandular elements, when present.30 Molecular/Genetics
Diagnostic criteria for the Carney complex include having 2 of 12 recognized clinical manifestations or 1 clinical manifestation plus evidence of genetic transmission, which is usually autosomal dominant.37 Two genetic loci have been ascribed to the Carney complex through linkage studies: 2p16 and 17q22-24.38 The cellular mechanism of cardiac myxoma development has yet to be elucidated. The clinical manifestation of the Carney complex is as follows:Tumor Spread and Staging
Myxomas can recur locally and spread to distant sites through embolization. Embolization appears to be much more likely in myxomas that are friable with a broad-based attachment and much less likely in fibrotic or calcified tumors.10 A recent study suggests that the expression of the mucin gene MUC5AC within sporadic cardiac myxomas may correlate with a lower risk of embolization.38 After embolization, myxomas are capable of infiltrating into the arterial wall and producing histologically identical tumors at the embolization site. Such lesions have been described in the skin, central nervous system, lungs, and bone.42,43,44,45
Given their benign nature, no official staging system exists for cardiac myxomas.Prognosis and Predictive Factors
Differentials
Calcified Amorphous Tumor of the Heart (Cardiac CAT) Metastatic Cancer, Unknown Primary Site Fibroma Myxoid Sarcoma Intracavity Hemangioma Papillary Fibroelastoma Mesothelial Incidental Cardiac Excrescence (MICE) Thrombus Multimedia

Media file 1: This gross photograph is an excellent example of a sessile myxoma arising within the left atrium. Note how the myxoma has a very broad attachment to the underlying myocardium. 
Media file 2: This gross photograph shows a sizable myxoma arising in the left atrium adjacent to the mitral valve. The tumor has been bivalved to demonstrate its variegated appearance. Clearly seen are pearly white, pale-gray, and yellow areas of tissue punctuated by patchy dark-red hemorrhage. 
Media file 3: This microscopic image shows a so-called "myxoma cell." Myxoma cells are frequently stellate with eosinophilic cytoplasm and indistinct cell borders. Their ovoid nuclei are typically pale with open chromatin. 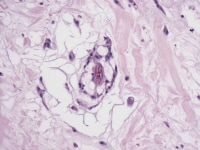
Media file 4: This photomicrograph demonstrates myxoma cells forming rings that are in close association with capillaries. 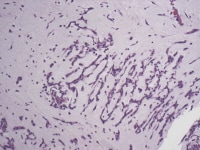
Media file 5: This photomicrograph demonstrates myxoma cells forming cords within a myxoid background. 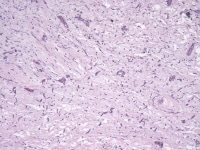
Media file 6: This photomicrograph shows myxoma cells forming rings, cords, and nests throughout the tumor. 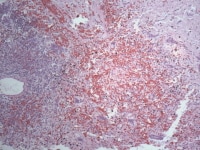
Media file 7: Prominent intratumoral hemorrhage can be seen in this photomicrograph and correlates with the dark-red hemorrhagic areas seen grossly. 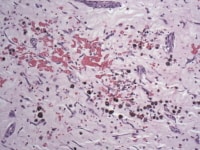
Media file 8: This photomicrograph demonstrates erythrocyte extravasation with accompanying hemosiderin deposition, indicating that the hemorrhage occurred in vivo and is not an artifact of processing. 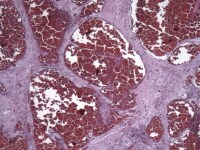
Media file 9: This photomicrograph demonstrates the numerous cavernous, thin-walled blood vessels that can be seen throughout the tumor. 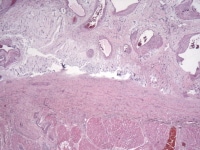
Media file 10: Larger, thick-walled blood vessels are frequently located near the pedicle or attachment of the myxoma to the underlying myocardium, as can be seen in this photomicrograph. 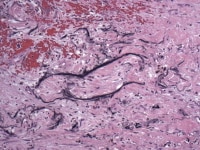
Media file 11: This photomicrograph shows the appearance of Gamma-Gandy bodies, which are calcific/siderotic elastic fibers often found within cardiac myxomas. 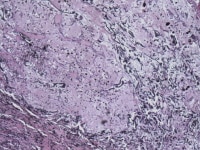
Media file 12: This photomicrograph demonstrates rather prominent calcific/siderotic elastic fiber degeneration, the so-called Gamna-Gandy body formation. 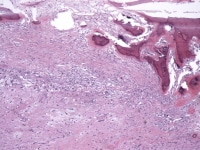
Media file 13: This photomicrograph demonstrates heterologous bone formation within the tumor. 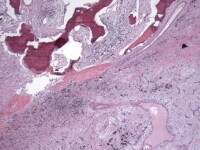
Media file 14: This photomicrograph demonstrates heterologous bone formation (upper left) within the tumor. The bottom right of the image shows classic myxoma cells in a myxoid background.
Sunday, October 17, 2010
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
Labels: Cardiac Myxoma
Subscribe to:
Post Comments (Atom)
0 comments:
Post a Comment