Atrial fibrillation (AF) is a supraventricular tachyarrhythmia characterized by disorganized atrial electrical activity and progressive deterioration of atrial electromechanical function. Electrocardiographic manifestations of atrial fibrillation include absence of sinus P waves; rapid oscillations (or fibrillatory [f] waves) that vary in amplitude, frequency, and shape; and an irregular ventricular response.
Overall, approximately 15-25% of all strokes in the United States (75,000/y) can be attributed to atrial fibrillation. Known risk factors for stroke in patients with atrial fibrillation include male sex, valvular heart disease (rheumatic valvular disease), heart failure, hypertension, and diabetes. Additional risk factors, such as advanced age and prior history of stroke, diabetes, and hypertension, place patients with preexisting atrial fibrillation at even higher risk for further comorbidities such as stroke (see Table 1).3
Open table in new window Unlike most cardiovascular diseases, the prevalence of atrial fibrillation is increasing in the United States and worldwide. Atrial fibrillation is frequently encountered in both the inpatient and outpatient settings. Primary therapeutic goals include rate control, maintenance of sinus rhythm, and prevention of thromboembolism.
While the precise mechanisms that cause atrial fibrillation are incompletely understood, atrial fibrillation appears to require both an initiating event and a permissive atrial substrate. Significant discoveries in the last decade have highlighted the importance of focal pulmonary vein triggers, but alternative and nonmutually exclusive mechanisms have also been evaluated. These include multiple wavelets, mother waves, fixed or moving rotors, and macro-reentrant circuits. In a given patient, multiple mechanisms may be present at any given time. The automatic focus theory and the multiple wavelet hypothesis appear to have the best supportive data.
The multiple wavelet hypothesis proposes that fractionation of wavefronts propagating through the atria results in self-perpetuating “daughter wavelets.” In this model, the number of wavelets is determined by the refractory period, conduction velocity, and mass of atrial tissue. In this model, increased atrial mass, shortened atrial refractory period, and delayed intra-atrial conduction increase the number of wavelets and promote sustained atrial fibrillation. This model is supported by data from patients with paroxysmal atrial fibrillation demonstrating that widespread distribution of abnormal atrial electrograms predicts progression to persistent atrial fibrillation.4 Intra-atrial conduction prolongation has also been shown to predict recurrence of atrial fibrillation.5 Together, these data highlight the importance of atrial structural and electrical remodeling in the maintenance of atrial fibrillation.
Atrial fibrillation affects more than 2.2 million Americans. One in 4 individuals 40 years of age and older will develop atrial fibrillation during their lifetime.6 Atrial fibrillation can occur in the absence of comorbidities, as it does in 10-15% of cases of atrial fibrillation (lone atrial fibrillation). However, atrial fibrillation is often associated with other cardiovascular diseases, including hypertension; heart failure; diabetes; ischemic heart disease; and valvular,dilated, hypertrophic, restrictive, and congenital cardiomyopathies.6
Atrial fibrillation is associated with increased morbidity and mortality, in part due to the risk of thromboembolic disease in atrial fibrillation and its associated risk factors. Disruption of normal atrial electromechanical function in atrial fibrillation leads to blood pooling and blood stasis. This, in turn, can lead to development of thrombus, most commonly in the left atrial appendage. Dislodgement of a clot can lead to embolic phenomena, including stroke.
Open table in new window
Open table in new window
Atrial fibrillation is strongly age-dependent, affecting 4% of individuals older than 60 years and 8% of persons older than 80 years. The rate of ischemic stroke among elderly patients not treated with warfarin averages approximately 5% per year.
Initial evaluation of the patient with new-onset atrial fibrillation should focus on the patient's hemodynamic stability. An effort should also be made to evaluate for potential comorbid diseases that contribute to initiation or maintenance of atrial fibrillation. Immediate electrical cardioversion should be considered for patients with hemodynamic collapse or evidence of cardiac ischemia.
The physical examination is helpful in determining underlying causes and sequelae of atrial fibrillation. An initial examination of the patient with new-onset atrial fibrillation should attend particularly to their hemodynamic stability.
Atrial fibrillation is strongly associated with established cardiovascular risk factors and advancing age. Hypertension, diabetes, and coronary artery disease promote atrial fibrillation. Structural heart disease, including valvular and congenital heart disease, is also associated with atrial fibrillation. Acute pulmonary processes, acute or chronic alcohol use (ie, holiday or Saturday night heart, also known as alcohol-related cardiomyopathy), illicit drug use (ie, stimulants, methamphetamines, cocaine) and hyperthyroidism also increase the risk of atrial fibrillation. Patients undergoing cardiothoracic or esophageal surgery are another population at risk for atrial fibrillation. In all, 20-40% of these patients experience postoperative atrial fibrillation. Certain poorly defined genetic factors may also contribute to an individual's propensity to develop atrial fibrillation.
Electrical cardioversion
Chemical cardioversion Several classification schemas have been proposed for the study of atrial fibrillation, but none fully accounts for all aspects of atrial fibrillation. A number of different labels and nomenclature have been used to describe patterns of atrial fibrillation, including acute, chronic, paroxysmal, intermittent, and permanent. The vagaries of each of these definitions make comparing the results of studies assessing the magnitude and treatment of atrial fibrillation difficult. Published guidelines from expert committees of the American College of Cardiology/American Heart Association and European Society of Cardiology on the treatment of patients with atrial fibrillation suggest that atrial fibrillation be classified into 3 patterns (see Media file 2). These include a first detectable episode, irrespective of whether it is symptomatic or self-limited, also recognizing that there may be some uncertainty about the duration of the episode and any prior undetected episodes. Recurrent atrial fibrillation is considered to be present when a patient has 2 or more episodes of atrial fibrillation. If atrial fibrillation terminates spontaneously, then recurrent atrial fibrillation is designated as paroxysmal; if this arrhythmia becomes sustained, then atrial fibrillation is considered persistent (irrespective of whether atrial fibrillation is terminated with pharmacologic therapy or electrical cardioversion). Persistent atrial fibrillation may be either the first presentation of atrial fibrillation or the result of recurrent episodes of paroxysmal atrial fibrillation. Patients with persistent atrial fibrillation also include patients with long-standing atrial fibrillation in whom cardioversion has not been indicated or attempted, often leading to permanent atrial fibrillation. Permanent atrial fibrillation is recognized as the accepted rhythm, and the main treatment goals are rate control and anticoagulation. While it is possible to reverse the progression from paroxysmal to persistent and to permanent, this task can be challenging.
Some patients with paroxysmal atrial fibrillation, typically younger patients, have been found to have distinct electrically active foci within their pulmonary veins. These patients generally have many atrial premature beats noted on Holter monitoring. Isolation or elimination of these foci can lead to elimination of the trigger for paroxysms of atrial fibrillation. Patients can also have atrial fibrillation as a secondary arrhythmia associated with cardiac disease that affects the atria (eg, congestive heart failure, hypertensive heart disease, rheumatic heart disease, coronary artery disease). These patients tend to be older, and atrial fibrillation is more likely to be chronic. Paroxysmal atrial fibrillation may progress to permanent atrial fibrillation, and aggressive attempts to restore and maintain sinus rhythm may prevent comorbidities associated with atrial fibrillation. Persistent atrial fibrillation with an uncontrolled, rapid ventricular heart rate response can cause a dilated cardiomyopathy and can lead to electrical remodeling in the atria (atrial cardiomyopathy). Therapy, such as drugs or atrioventricular nodal ablation and permanent pacemaker implantation, to control the ventricular rate can improve left ventricular function and improve quality-of-life scores. New developments aimed at curing atrial fibrillation are being actively explored. By reducing the critical mass required to sustain atrial fibrillation with either surgical or catheter-based compartmentalization of the atria (ie, MAZE procedure), fibrillatory wavelets collide with fixed anatomic obstacles, such as suture lines or complete lines of ablation, thus eliminating or reducing the chance of chronic atrial fibrillation. Some patients with focal origins of their atrial fibrillation also may be candidates for catheter ablation. Still, much remains to be accomplished before either of these procedures is appropriate for primary treatment. Management of new-onset atrial fibrillation differs from that of long-term atrial fibrillation.
However, several randomized controlled trials have demonstrated that a strategy aimed at restoring (and maintaining) sinus rhythm neither improves the survival rate nor reduces the risk of stroke in patients with atrial fibrillation.
Rate control
Anticoagulation
Effective anticoagulation in patients with atrial fibrillation reduces the risk of stroke 3-fold. Patients with newly diagnosed atrial fibrillation and patients awaiting electrical cardioversion can be started on intravenous heparin (activated partial thromboplastin time [aPTT] of 45-60 s) or low molecular weight heparin (1 mg/kg bid).
Oral direct thrombin inhibitors may represent an alternative to warfarin in a higher-risk population with nonvalvular atrial fibrillation, but no agents in this class are currently approved in the United States. In the highest-risk population (eg, atrial fibrillation with valvular heart disease or prior embolic cerebrovascular accident) bridging anticoagulation with heparins may be required in the periprocedural period.
Long-term management of atrial fibrillation is focused on reducing the likelihood of atrial fibrillation recurrence, reducing atrial fibrillation-related symptoms, control of ventricular rate, and reducing stroke risk. As discussed previously, atrial fibrillation often results from exposure to established cardiovascular risk factors. Appropriate management of these risk factors will reduce the likelihood of future atrial fibrillation and atrial fibrillation—related morbidity and mortality. Anticoagulation should be initiated for all individuals with atrial fibrillation with either aspirin or warfarin except those with "lone" atrial fibrillation or contraindications. Selection of the appropriate antithrombotic drug should be based on the risk of stroke and bleeding for a given patient. Antiarrhythmic therapy can aid in maintenance of sinus rhythm in certain patients but requires close monitoring.
A study by van Walraven et al determined that as patients with atrial fibrillation age, the relative efficacy of oral anticoagulation does not decrease, whereas the efficacy of antiplatelet therapy does appear to decrease as a patient ages.15 Rate control
Every effort should be made to assess effectiveness of rate control both at rest and with exertion, especially in those patients who primarily experience exertional atrial fibrillation-related symptoms. Twenty-four hour Holter monitoring or exercise-treadmill testing can be helpful in evaluating heart rate variability. Adequate rate control can be defined as a heart rate of 60-80 bpm at rest and 90-115 bpm with moderate exercise. Since its inception, surgical compartmentalization of the atria, or the MAZE procedure, has evolved as an exciting procedure with a potential to cure atrial fibrillation. Quite simply, the atria are transected and resutured to reduce the critical mass required for the maintenance of atrial fibrillation. Early experience shows that atrial transport is restored postoperatively and that long-term anticoagulation is not required. The downside remains the need for an open chest procedure; however, thoracoscopic procedures may reduce hospitalization and recovery times in the future. The surgical MAZE procedure remains an attractive procedure for patients with atrial fibrillation who are undergoing concomitant mitral valve procedures. Its role as a primary therapy for atrial fibrillation is doubtful.
Consultation with a cardiac electrophysiologist or knowledgeable clinician is recommended prior to antiarrhythmic drug initiation. Diet restrictions, if any, are as appropriate for the underlying heart disease and any other comorbidities (eg, diabetes mellitus). The goals of medical therapy for patients with atrial fibrillation are to maintain sinus rhythm, avoid the risk of complications (eg, stroke), and minimize symptoms. Warfarin represents the cornerstone of anticoagulant therapy for patients at moderate to high-risk of thromboembolic events.
If maintenance of sinus rhythm is the goal, the ACA/AHA/ECC have jointly developed guidelines for the long-term antiarrhythmic treatment in the maintenance of sinus rhythm.3 The following algorithm incorporates clinical trial data on the safety and efficacy of antiarrhythmic agents. These guidelines are intended to help clinicians tailor antiarrhythmic therapy on an individual basis for their patients.
Used to slow ventricular response by slowing AV nodal conduction during atrial fibrillation or atrial flutter. Also indicated for use in conjunction with class IA and IC antiarrhythmics, which slow atrial fibrillation/flutter rate and may cause more rapid ventricular response.Introduction
Background
Atrial fibrillation is the most common arrhythmia encountered in clinical practice (see Media file 1) and is a significant public health problem in the United States. Atrial fibrillation affects more than 2.2 million Americans and almost 5% of the population older than 69 years. The prevalence of atrial fibrillation increases dramatically with age. Atrial fibrillation is associated with known cardiovascular risk factors such as hypertension, coronary artery and valvular heart disease, heart failure (HF) and diabetes mellitus.1
Data from the Framingham heart study show that atrial fibrillation is associated with a 1.5- to 1.9-fold higher risk of death, which is in part due to the strong association between atrial fibrillation and thromboembolic events.2 While patients can be asymptomatic, many experience a wide variety of symptoms, including palpitations, dyspnea, fatigue, dizziness, angina, and decompensated heart failure. In addition, atrial fibrillation can be associated with hemodynamic dysfunction, tachycardia-induced cardiomyopathy, and systemic thromboembolism.
Table 1. Risk Factors for Stroke in Patients with Nonvalvular Atrial Fibrillation
Risk Factors
Relative Risk
Prior stroke or TIA
2.5
History of hypertension
1.6
Heart failure and/or reduced left ventricular function
1.4
Advanced age
1.4
Diabetes
1.7
Coronary artery disease
1.5
Patients with rheumatic heart disease and atrial fibrillation have an even higher risk for stroke (17-fold). At least 4 large clinical trials have clearly demonstrated that anticoagulation with warfarin decreases the risk of stroke by 50-80%.
For related information, see Medscape's Atrial Fibrillation Resource Center.
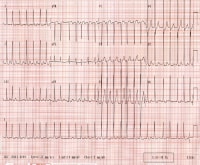
Ventricular rate varies from 130-168 beats per minute. Rhythm is irregularly irregular. P waves are not discernible.
Pathophysiology
A focal origin of atrial fibrillation is supported by several experimental models showing that atrial fibrillation persists only in isolated regions of atrial myocardium. This theory has garnered considerable attention recently as studies have demonstrated that a focal source of atrial fibrillation can be identified in humans and that isolation of this source can eliminate atrial fibrillation.
The pulmonary veins appear to be the most frequent source of these automatic foci, but other foci have been demonstrated in several areas throughout the atria. Cardiac muscle in the pulmonary veins appears to have active electrical properties similar, but not identical, to those of atrial myocytes. Heterogeneity of electrical conduction around the pulmonary veins is theorized to promote reentry and sustained atrial fibrillation. Thus, pulmonary vein automatic triggers may provide the initiating event and heterogeneity of conduction may provide the sustaining event in many patients with atrial fibrillation.
Atrial fibrillation shares strong epidemiologic associations with other cardiovascular diseases such as heart failure, coronary artery disease, valvular heart disease, diabetes mellitus and hypertension.1 These factors have been termed upstream risk factors, but the relationship between comorbid cardiovascular disease and atrial fibrillation is incompletely understood and more complex than this terminology implies. The exact mechanisms via which cardiovascular risk factors predispose to atrial fibrillation are not fully understood but are under intense investigation. Catecholamine excess, hemodynamic stress, atrial ischemia, atrial inflammation, metabolic stress, and neurohumoral cascade activation are all purported to promote atrial fibrillation.Frequency
United States
Atrial fibrillation can be triggered after cardiac surgery and is associated with pulmonary disease, thyrotoxicosis, acute ethanol intoxication, and electrolyte imbalance. Given the almost epidemic proportions of patients with atrial fibrillation, clinicians should be aware of the multiple mechanisms and triggers for atrial fibrillation. Correcting the underlying disorder is often necessary to successfully treat atrial fibrillation.
Mortality/Morbidity
One of the major management decisions in atrial fibrillation (and atrial flutter) is determining the risk of stroke and appropriate anticoagulation regimen for low-, intermediate-, and high-risk patients. For each anticoagulant, the benefit in terms of stroke reduction must be weighed against the risk of serious bleeding.
Most clinicians agree that the risk-benefit ratio of warfarin therapy in low-risk patients with atrial fibrillation is not advantageous (due to the increased risk of a significant bleed versus the risk of stroke in low-risk patients). Warfarin therapy has, however, been shown to be beneficial in higher-risk patients with atrial fibrillation. A target international normalized ratio (INR) of 2-3 is traditionally used in this cohort as this limits the risk of hemorrhage, while providing protection against thrombus formation.
The appropriate treatment regimen for patients with atrial fibrillation at intermediate risk is controversial. In this population, the clinician should assess risk factors for thromboembolic disease, patient preference, risk of bleeding, risk of falls or trauma, and likelihood of medication adherence. Warfarin is also superior to clopidogrel or a combination of clopidogrel and aspirin in the prevention of embolic events in higher-risk patients. A new class of oral direct thrombin inhibitors are in the late stages of clinical trial or pending approval and may be as effective and as safe as warfarin in higher-risk nonvalvular atrial fibrillation.
Several risk factor assessment algorithms have been developed to aid the clinician in decision-making regarding anticoagulation in atrial fibrillation. The CHADS2 index7 (Cardiac failure, Diabetes, Stroke [or S2 = TIA]) is the most widely used of these algorithms. The CHADS2 index uses a point system to determine yearly thromboembolic risk. Two points are assigned for a history of stroke or TIA, and one point is given for age over 75 or a history of hypertension, diabetes, or heart failure. The predictive value of this scoring system was evaluated in 1733 elderly patients with nonvalvular atrial fibrillation aged 65-95 who were not given warfarin at hospital discharge. Although high scores were associated with an increased rate of stroke, few patients had a score greater than 5 or a score of 0 (see Table 2).
Table 2. Adjusted Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with Anticoagulation
CHADS2 Score
Adjusted Stroke Rate (%/y)
0
1.9
1
2.8
2
4.0
3
5.9
4
8.5
5
12.5
6
18.2
Recommendations for anticoagulation for patients with nonvalvular atrial fibrillation are based on 2006 ACC/AHA/ESC task force guidelines on the management of patients with atrial fibrillation8 (see Table 3).
Table 3. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial FibrillationRisk Category
Recommended Therapy
No risk factors
Aspirin 81-325 mg daily
One moderate-risk factor
Aspirin 81-325 mg daily or warfarin (INR 2-3)
Any high-risk factor or more than 1 moderate-risk factor
Warfarin (INR 2-3)
High-risk factors include prior stroke, TIA, and systemic thromboembolism.
Moderate-risk factors include age older than 75 years, hypertension, heart failure, left ventricular function <35%, and diabetes mellitus.
Risk factors of unknown significance include female gender, age 65-74 years, coronary artery disease, and thyrotoxicosis.Age
Clinical
History
Physical
Causes
Laboratory Studies
Imaging Studies
Other Tests
Procedures
Staging
This classification schema pertains to cases that are not related to a reversible cause of atrial fibrillation (eg, thyrotoxicosis, electrolyte abnormalities, acute ethanol intoxication). The occurrence of atrial fibrillation secondary to acute myocardial infarction, cardiac surgery, pericarditis, pulmonary embolism, or acute pulmonary disease is considered separately because, in these situations, atrial fibrillation is less likely to recur once the precipitating condition has been resolved and adequately treated.
Treatment
Medical Care
Management of New-Onset Atrial Fibrillation
The management of atrial fibrillation can be broken down into management of new-onset and long-standing atrial fibrillation. The cornerstones of new-onset atrial fibrillation management are rate control and anticoagulation.12 The clinical decision to use a rhythm control or rate control strategy requires integration of several factors, including degree of symptoms, likelihood of successful cardioversion and presence of comorbidities. Anticoagulation is an important consideration in both new onset and long-standing atrial fibrillation. See Media file 3.
Restoration of sinus rhythm with regularization of the heart's rhythm improves cardiac hemodynamics and exercise tolerance. By maintaining the atrial contribution to cardiac output, symptoms of heart failure and overall quality of life can improve. As atrial fibrillation contributes to pathologic atrial and ventricular remodeling, restoration of sinus rhythm can slow and, in some cases, reverse atrial dilatation and left ventricular dysfunction. For these reasons, most clinicians focus initially on maintenance of sinus rhythm and opt for a rate control strategy only when rhythm control fails.
In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study,13 4060 subjects aged 65 years or older whose atrial fibrillation was likely to be recurrent and who were at risk for stroke were randomized to a strategy of rhythm control (cardioversion to sinus rhythm plus drugs to maintain sinus rhythm) versus a strategy of rate control (in which no attempt was made to restore or maintain normal sinus rhythm). An insignificant trend toward increased mortality was noted in the rate control group, and, importantly, no evidence suggested that the rhythm control strategy protected patients from stroke. Clinically silent recurrences of atrial fibrillation in the rhythm control group are theorized to be responsible for the increased rates of thromboembolic events and mortality noted in this cohort. This underscores the importance of anticoagulation in both rhythm control and rate control patients.
The AFFIRM study (and similar findings from the smaller Rate Control Versus Electrical Cardioversion [RACE] trial14 ) has led to the development of consensus guidelines that recommend an initial rate-control strategy for many asymptomatic patients with atrial fibrillation. The ACC/AHA/ESC 2006 guidelines state that an initial rate control strategy is "reasonable" for asymptomatic or minimally symptomatic older patients with hypertension and comorbid cardiovascular disease.3 These same guidelines state that for younger individuals, especially those without significant comorbid cardiovascular disease, an initial rhythm control strategy may be a better approach.
Regardless of long-term strategy chosen, control of ventricular rate is a critical component of management of new-onset atrial fibrillation. The main determinants of the ventricular rate during atrial fibrillation are those intrinsic and extrinsic factors that influence atrioventricular (AV) conduction. Foremost among these are the intrinsic AV nodal conduction properties. Underlying sympathetic and parasympathetic tone also influences AV nodal conduction. Rate-controlling agents primarily act by increasing AV nodal refractoriness.
Atrial fibrillation is recognized as a powerful risk factor for stroke. One of the most important considerations in the acute management of atrial fibrillation is the need for anticoagulation. Acute cardioversion for atrial fibrillation carries a risk of thromboembolism unless anticoagulation therapy is initiated prior to the procedure and continued post-procedure. Risk of thromboembolism in patients undergoing either pharmacologic or electrical cardioversion is similar. The risk of thromboembolic events is greatest when atrial fibrillation has been present for longer than 48 hours.
Patients can be concomitantly started on warfarin in an inpatient setting while awaiting a therapeutic INR value (2-3). Many practices have developed specialized anticoagulation clinics to closely monitor INR values.
Cardioversion
Cardioversion may be performed electively or emergently to restore sinus rhythm in patients with new-onset atrial fibrillation. Cardioversion is most successful when initiated within 7 days after to onset of atrial fibrillation. The need for cardioversion may be acute when atrial fibrillation is responsible for hypotension, heart failure, or angina.
Pharmacologic agents or direct current energy can be used to cardiovert patients with atrial fibrillation. Pharmacologic cardioversion has the advantage of not requiring sedation or anesthesia, but the major disadvantage is the risk of ventricular tachycardia and other serious arrhythmias.
Long-Term Management of Atrial Fibrillation
Decision-making with regard to the optimal long-term strategy for atrial fibrillation management should be based on a thorough integration of patient-specific factors and likelihood of success. As a rule, younger patients with more severe symptoms and fewer comorbidities tend to derive a greater benefit from a long-term focus on rhythm control. Older patients with structural heart disease (ie, left ventricular hypertrophy, prior myocardial infarction, depressed ejection fraction, or atrial dilation) are less likely to remain in sinus and are more likely to have serious side-effects from antiarrhythmic drugs. In this cohort, most clinicians focus on long-term rhythm control.
Atrial fibrillation causes electrophysiologic and structural remodeling which, in turn, promotes future atrial fibrillation ("atrial fibrillation begets atrial fibrillation"). As such, many patients with paroxysmal atrial fibrillation will progress to persistent and permanent atrial fibrillation. The degree to which this reflects the continuing influence of underlying cardiovascular risk factors as opposed to a direct effect of atrial fibrillation is unknown. Regardless, clinicians frequently need to reevaluate their management strategies as atrial fibrillation burden and comorbidities increase with time.
Anticoagulation
The goal of long-term anticoagulation in atrial fibrillation is to reduce the risk of thromboembolism.
As discussed previously, several trials have validated the noninferiority of an initial rate-control strategy. Many clinicians believe, however, that an attempt at a rhythm control strategy should be made in most patients. Older patients with comorbid cardiovascular disease have a lower likelihood of successful long-term rhythm control and thus these patients are often managed using a rate-control strategy. Some patients initially managed with a rhythm control strategy will experience progression to recurrent or persistent atrial fibrillation. Clinicians often switch to a rate control strategy as the atrial fibrillation burden increases.
Maintenance of sinus rhythm requires treatment of cardiovascular risk factors and any underlying disorder (ie, hyperthyroidism) that may have triggered atrial fibrillation. As discussed previously, several antiarrhythmic drugs (flecainide, propafenone, dofetilide, amiodarone) have established efficacy in the pharmacologic conversion of atrial fibrillation to sinus rhythm.
A study by Doyle and Ho determined that amiodarone, as a part of a strategy to achieve sinus rhythm, appears safe and effective in patients with persistent atrial fibrillation. However, intolerable adverse effects were more common in amiodarone than placebo or rate control drug.16
Several distinct agents, most notably sotalol, dofetilide, and dronedarone, are used for the long-term maintenance of sinus rhythm. Sotalol is efficacious but, like other Class III drugs, requires close monitoring of the QT interval and serum electrolytes. Unlike dofetilide and amiodarone, sotalol is contraindicated in patients with structural heart disease and heart failure. Dofetilide is efficacious in maintaining sinus rhythm but requires admission to a hospital in a monitored setting for initiation. The drug is renally cleared and dosing is based on glomerular filtration rate. Dronedarone has recently gained approval for the maintenance of sinus rhythm, but its efficacy is below that of amiodarone. While safer (ie, no negative effects on thyroid, pulmonary, or liver function), it is contraindicated in patients with Class IV heart failure and recently decompensated Class II and Class III heart failure.
Catheter ablation is an alternative to pharmacologic therapy to prevent recurrent atrial fibrillation in symptomatic patients.17 Catheter ablation is currently being performed in select centers for paroxysmal and persistent atrial fibrillation and has become a second line of therapy after drug failure or drug intolerance. Surgical ablation of atrial fibrillation is also an option for patients with atrial fibrillation, especially those undergoing other cardiac surgery and in those patients in whom pharmacologic and catheter-based procedures are ineffective or contraindicated. Atrial fibrillation ablation may be superior to AV nodal ablation and biventricular pacing in heart failure patients but is technically difficult and demanding, and the widespread applicability of ablation in this population of patients is uncertain.
New medical and device-based rhythm control therapies are being actively explored. Experimental and clinical data suggest that renin-angiotensin system (RAS) antagonists and HMG-CoA-Reductase Inhibitors (statins) may decrease the incidence of atrial fibrillation and increase the likelihood of successful cardioversion.18,19,20,21Device-based therapies under research include single- and dual-site atrial pacemakers to prevent atrial fibrillation and atrial defibrillators to rapidly restore sinus rhythm. Invasive (surgical and catheter-based) therapies to compartmentalize the atria and localize focal triggers (in the pulmonary veins) are being evaluated and refined. (See Surgical Care.)
Special considerations
Postoperative atrial fibrillation is common (20-30%) and perioperative beta-blockers are recommended in all patients undergoing cardiac surgery unless contraindicated.22 Preoperative administration of amiodarone and sotalol may reduce the incidence of atrial fibrillation in patients undergoing cardiac surgery. As such, these agents may be used as prophylactic therapy in those at high risk for postoperative atrial fibrillation. Unless a preoperative diagnosis, most postoperative atrial fibrillation is the result of tissue irritation and will have resolved by the sixth postoperative week.
Retrospective data suggest that atrial-based pacing (AAI, DDD modes) reduces the risk of developing atrial fibrillation and increases the interval between episodes in patients with sick sinus syndrome.23Surgical Care
Catheter ablation has taken the following 3 paths in the attempt to cure or manage atrial fibrillation. Despite classification of their atrial fibrillation (paroxysmal, persistent, permanent) patients undergoing catheter ablation have been shown to have less atrial fibrillation than control groups treated with antiarrhythmic agents.24,25,26
Roux et al studied whether empiric therapy with antiarrhythmic drugs (AAD) following atrial fibrillation (AF) ablation would decrease the incidence of atrial arrhythmias that commonly occur following ablative therapy. Patients undergoing AF ablation were randomized to receive empiric AAD therapy or no AAD therapy for the initial 6 weeks after ablation. Measured outcomes included atrial arrhythmias lasting more than 24 hours; atrial arrhythmias associated with severe symptoms that required hospitalization, cardioversion, or initiation/change of antiarrhythmic drug therapy; and intolerance to antiarrhythmic agent requiring drug cessation. Results showed AAD treatment after AF ablation was well tolerated and reduced the incidence of clinically significant atrial arrhythmias and the need for cardioversion or hospital admission.32Consultations
Diet
Medication
Some patients may not be able to take anticoagulants because of contraindications or comorbidities. The ACTIVE trial studied 7554 patients with atrial fibrillation with the intent to determine if adding clopidogrel to aspirin therapy would reduce the risk for acute vascular events (ie, stroke, myocardial infarction, non-CNS systemic embolism, or death from vascular event) in patients unable to take warfarin. Addition of clopidogrel to aspirin reduced the risk of major vascular events (P=0.01), especially stroke (P=0.001), compared with placebo and aspirin. Increased risk for major hemorrhage was more prevalent in the clopidogrel plus aspirin group than the placebo and aspirin group.33
The goal of antiarrhythmic drug therapy is to reduce the duration and frequency of atrial fibrillation episodes, thus improving patient quality of life and reducing symptoms.
Several antiarrhythmic drugs are commonly used to prevent atrial fibrillation recurrence. Currently, the FDA has approved 6 antiarrhythmic drugs (quinidine, flecainide, propafenone, sotalol, dofetilide, dronedarone) for the treatment of atrial fibrillation. Other antiarrhythmic agents (eg, amiodarone) are used in an off-label fashion with great clinical efficacy. Use of antiarrhythmic drugs requires caution because they can also be proarrhythmic. These agents can exacerbate pre-existing arrhythmias and generate arrhythmia de novo. Tachy- and brady-arrhythmias generated by these agents can be of ventricular or atrial origin. Drug-drug interactions and extra-cardiac side effects are common. Consultation with a cardiac electrophysiologist or knowledgeable clinician is recommended prior to antiarrhythmic drug initiation.
For patients with no evidence of structural heart disease, flecainide, propafenone, sotalol, dronedarone, or dofetilide should be considered first-line agents. Amiodarone can be considered as alternative agents. For patients with substantial left ventricular hypertrophy (LVH), amiodarone is considered a reasonable first-line agent. For patients with coronary artery disease, dofetilide or sotalol are first-line therapy. Amiodarone is considered a second-line agent in this population. For patients with heart failure, amiodarone or dofetilide are first-line agents. Dronedarone should not be used in patients with Class IV heart failure or in Class II-III patients with recent heart failure exacerbations. See Media file 4.
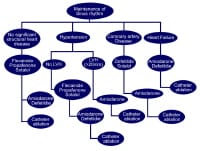
Antiarrhythmic drug algorithm for the medical management of sinus rhythm in patients with atrial fibrillation.
Current practice constraints mandate that clinicians carefully consider patient populations at low and acceptable risks for outpatient antiarrhythmic drug initiation. Proarrhythmia is the most common adverse effect of antiarrhythmics during the loading phase. While the proarrhythmic effect of these drugs extends into the maintenance phase, inpatient drug initiation is generally recommended in the monitored inpatient setting, especially for those patients with structural heart disease or substantial comorbidities. Nevertheless, certain antiarrhythmic drugs have established and acceptable safety profiles when used in outpatients without structural heart disease or other risk factors.Atrioventricular nodal conduction blockers
Further Inpatient Care
Further Outpatient Care
Deterrence/Prevention
Prognosis
Multimedia

Media file 1: Ventricular rate varies from 130-168 beats per minute. Rhythm is irregularly irregular. P waves are not discernible. 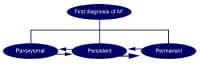
Media file 2: Classification scheme for patients with atrial fibrillation. 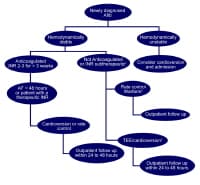
Media file 3: Patient management for newly diagnosed atrial fibrillation. Subtherapeutic INR: INR <2> 
Media file 4: Antiarrhythmic drug algorithm for the medical management of sinus rhythm in patients with atrial fibrillation.
Sunday, October 17, 2010
Labels: Atrial Fibrillation
Subscribe to:
Post Comments (Atom)
0 comments:
Post a Comment