Transplantation is a burgeoning and rapidly evolving science. There continues to be much to learn from autopsies of transplant recipients. The United Network for Organ Sharing maintains adatabase of the cause of death of all transplant recipients, and autopsies can contribute importantly to the accuracy of that database as well as to the practice of transplantation at individual transplant centers.1Pathologists at hospitals with a high volume of transplantations are familiar with transplant pathology, especially the microscopic pathology, and there are entire books devoted to the subject, with increasing devotion to the microscopic pathology.2 In addition to all the natural diseases and complications any person could have, transplant recipients can have complications of the surgical transplantation procedure, rejection of the transplanted organ, and complications ofposttransplant immunosuppression.3 An important part of doing a good autopsy of a particular transplant recipient is knowing what to pay most attention to. This requires a focused review of the medical record before doing the autopsy. Review the deceased's medical record for: The categories of disease that can be grossly visible at autopsy in transplant recipients include infections, rejection, neoplasms (particularly posttransplant lymphoproliferative disorder), reperfusion injury, graft-versus-host disease, and all the other diseases any patient can have. Knowing the type of transplant and type of transplant anastomoses, having some idea of the type and level of immunosuppression, being aware of the history of rejection (if any) and the history of infections (if any), knowing the clinician's perspective on the causes of death and other issues, as well as understanding the categories of disease that can be grossly visible at autopsy in transplant recipients -- including infections, rejection, posttransplant lymphoproliferative disorder, reperfusion injury, and graft-versus-host disease -- aid the autopsy prosector in being ready to perform the autopsy. If a pathologist who is unfamiliar with transplantation pathology is faced with doing the autopsy of a transplant recipient, the best approach is to focus on doing the optimum special dissections, obtaining the optimum microbiologic cultures, and taking the optimum microscopic sections. The slides can always be reviewed later by a pathologist with more specific expertise, but one cannot go back and take cultures or do special dissections later. Transplanted organs should be examined for rejection and infection with multiple microscopic sections. * Most of these sections need no explanation, but 2 merit it. The deep hilum section of a transplanted liver provides examination of the large perihilar arteries and bile ducts, which can demonstrate rejection, particularly chronic rejection.11 Similarly, the knuckle section of the left anterior descending coronary artery can demonstrate cardiac allograft vasculopathy.19 One way to think of the likelihood of finding vascular rejection in the knuckle is that one of the most likely places a recipient lymphocyte (capable of causing vascular rejection) will run into a donor endothelial cell is in the knuckle where the arterial blood flow hits the wall of the segment making a right-angle turn down into the myocardium. The categories of disease that can be visible in the microscopic sections from autopsies of transplant recipients include infections, rejection, neoplasms (particularly posttransplant lymphoproliferative disorder), reperfusion injury, graft-versus-host disease, and all the other diseases any patient can have. The most unique is transplant rejection. Microbiologic cultures are an important part of a transplant autopsy. There is no time limit for a postmortem interval between death and autopsy after which cultures become worthless, although a particularly long interval of 2 or more days may increase the incidence of postmortem "contaminants" in aerobic bacterial cultures. Such contaminants could conceivably overgrow a true pathogen that grows more slowly.Introduction
This chapter is devoted to what a pathologist unfamiliar with transplant pathology needs to know in order to do the best possible postmortem examination of a transplant recipient, focusing on the performance (preparation, dissection, and gross pathology), particularly those aspects that need to be done right in preparation or at the autopsy table, because poor performance cannot be subsequently undone. A pathologist unfamiliar with transplant pathology can send the microscope slides to another pathologist with more specific expertise to get the best possible microscopic diagnoses. This chapter will not seek to condense the knowledge of entire books into part of this brief review nor to provide what is best obtained from a review of the slides by an expert in transplant pathology.Preparation for the Autopsy of a Transplant Recipient
Pertinent information includes the type of transplant, type of disease for which transplantation was performed, type of transplant anastomoses, type and level of immunosuppression, history of rejection, and history of infections already diagnosed antemortem. The types of transplant include peripheral blood stem cell, kidney, liver, heart,lung, intestine, pancreas, and various combinations.
Transplantation of heart valves, corneas, or bone does not require immunosuppression, so autopsies of patients with such transplants do not require the search for transplant rejection and opportunistic infection important in the autopsies of the other types of transplant patients. Peripheral blood stem cell transplantation has replaced bone marrow transplantation, which should not be confused with bone transplants for orthopedic disease.
Transplant rejection has a continuously evolving and sometimes confusing terminology. It can be cell-mediated or antibody-mediated or, frequently, both. Rejection can be acute or chronic, but in the past, cell-mediated rejection, which can occur months or years following transplantation, was referred to as acute cellular rejection, no matter how long after transplantation it occurred, a source of confusion.
The classification of kidney transplant rejection has been updated using the terms "T-cell mediated rejection" and "antibody-mediated changes."4 Similarly, what was called "acute cellular rejection" in heart transplantation is now called simply "rejection."5 Cell-mediated rejection of lung transplants, however, is still called "acute rejection."6Details of the donor and recipient tissue cross-match can be important in determining the likelihood of rejection, but these can be looked up after performance of the autopsy.
The type of transplant affects the likelihood of infection. Stem cell transplant recipients, for instance, are particularly likely to have infection.7,8 Myeloablative conditioning for stem cell transplantation adds a risk ofneutropenia (from the conditioning or poor engraftment following conditioning) to the risk of immunosuppression. Recipients of any type of transplant who are seronegative for Epstein-Barr virus and receive organs from Epstein-Barr virus seropositive donors are at increased risk of posttransplant lymphoproliferative disorder. Blood testing for Epstein-Barr virus nucleic acid can reveal evidence of lymphoproliferative disorder antemortem.9
Recipients who are seronegative for cytomegalovirus and receive organs from cytomegalovirus seropositive donors are at increased risk of cytomegalovirus infection; blood testing for cytomegalovirus nucleic acid can provide evidence of this. Many transplant recipients develop neutropenia, sometimes as a side effect of the numerous medications they need, and this puts them at higher risk for all the infections typically more common in neutropenic patients.10
Some diseases for which transplantation are performed can occur in the transplanted organs. This is referred to as "recurrence" even though the disease was usually not present in the organ before transplantation. This process is especially common in transplanted livers. Hepatitis B (HBV) recurs in transplanted livers if HBV DNA was positive before transplantation, and hepatitis C (HCV) recurs in transplanted livers if HCV replication was occurring before transplantation.11 Sarcoidosis can recur in transplanted lungs.12 Knowing which diseases tend to recur in transplanted organs and which disease(s) an autopsy patient received a transplant for can be important in recognizing recurrent disease in transplanted organs.
It is always important to talk to the deceased's clinicians before an autopsy of their patient. If a long time has elapsed since transplantation, the most important clinician to speak to is usually a pulmonologist, a cardiologist, or some other nonsurgeon who was following the patient or managing the patient's acute care terminally. If the interval since transplantation is short, the most important clinician to communicate with is commonly the transplant surgeon. This is especially true if the patient dies in the immediate postoperative period. Inviting the surgeon to attend the autopsy is always a good idea, particularly if the anastomoses were complicated or if surgical bleeding is high in the differential diagnosis as the immediate cause of death.
In a case of primary allograft failure, the transplant surgeon is absolutely the essential clinician to speak to. In such a case, some of the most pertinent information is about the donor and donor organ, particularly a donor history of shock requiring vasopressor therapy and the "ischemic time," the length of time between removing the organ from the donor and reperfusing it in the recipient. The surgeon may also be able to provide other helpful details regarding the harvest and transportation and implantation of the donated organ.
Reperfusion of a transplanted organ in the recipient releases toxic metabolites that have accumulated during or before the ischemia time, and this may be associated with "reperfusion injury," which can be an important cause of graft malfunction or illness in the recipient. Knowing that there is a history of reperfusion injury is helpful in interpreting histopathologic evidence of tissue damage or necrosis -- and sometimes even in determining the cause of death.
One of the important things to know from the patient's clinical history is whether there was an episode of rejection that prompted increased immunosuppression or an episode of infection that prompted decreased immunosuppression in the period shortly before the patient died. A history of increased immunosuppression has the effect of making infection more likely at autopsy, and decreased immunosuppression makes rejection more likely.
The photograph below shows a fistula from the iliac artery to the jejunum at the site of a pancreas transplant withpancreatitis and infection at the site, which no doubt digested the tissue between the artery and the intestine. Knowing that the patient had presented with passing bright red blood per rectum allowed an efficient, focused autopsy dissection, which revealed the fistula. As a general principle, the more pertinent information an autopsy pathologist has before performing the autopsy, the easier it is to do a good autopsy.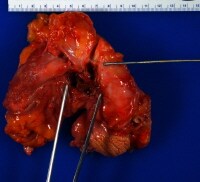 upper right, a...">
upper right, a...">Fistula from the iliac artery (upper right, above probe) to the jejunum (lower left, immediately above the forceps) at the site of a pancreas transplant.
Preparation Checklist for the Autopsy of a Transplant Recipient
Contact the clinicians to:Gross Examination and Findings
The skin of a transplant recipient may show ulcerations due to opportunistic infections such as herpes simplex virus (HSV), nodules due to opportunistic infections such as cryptococcosis, nodular and/or ulcerative neoplasms such as squamous cell carcinoma, or rashes, which may represent drug reactions or graft-versus-host disease. Graft-versus-host disease is most common in stem cell transplant patients, but this condition can occur in solid organ transplant recipients (due to "passenger lymphocytes" in the transplanted organ).
The first organ to have graft-versus-host disease is almost always the skin.13 Acute graft-versus-host disease takes the form of a maculopapular skin rash, most commonly starting in the third week following transplantation, but sometimes as late as 11 weeks post transplant. Chronic graft-versus-host disease produces indurated erythematous or violaceous papules or plaques, with a squamous surface, sometimes confluent, resembling lichen planus.14 Late chronic graft-versus-host disease is associated with a skin condition resembling systemic sclerosis(scleroderma).
Graft-versus-host disease sometimes involves the liver and mucosa of the gastrointestinal tract (including the portion shared with the respiratory tract). The hepatitis caused by graft-versus-host disease is not distinctive, and an affected liver may appear normal grossly or it may be softened and discolored. The inflammation caused by graft-versus-host disease in the gastrointestinal tract is associated with erythema or congestion, erosion, or ulceration, which may be hemorrhagic.
The gross pathology of reperfusion injury (harvest injury, preservation injury) is essentially the same as ischemic reperfusion injury outside the context of transplantation. A transplanted heart with sufficient reperfusion injury to cause primary graft failure may have hemorrhage visible from the reperfusion. A transplanted lung with reperfusion injury sufficient to cause primary graft failure will be heavy, consolidated, congested, and edematous. The color is sometimes described as "beefy red" and the consistency as slippery. A transplanted liver with severe reperfusion injury will likely be softened and congested.
In the internal organs, in addition to all the usual gross pathology of infections and other diseases, a nodule or mass lesion may be post transplant lymphoproliferative disorder.15 Posttransplant lymphoproliferative disorder is most common in whatever organ was transplanted, but this condition may occur in lymph nodes or anywhere else in the body. It may produce discrete lesions with the color and consistency of fish flesh, if it is fully evolved to a malignant lymphoma. Furthermore, it may have an off-white, creme, tan, pink, or even red color, with indistinct margins.
Posttransplant lymphoproliferative disorder may be present without being grossly visible. The image below shows a posttransplant lymphoproliferative disorder which had monomorphic diffuse large B-cell areas, in the intestine and adjacent lymph node, producing raised, bile-stained lesions in the intestinal mucosa.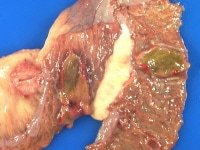
Posttransplant lymphoproliferative disorder in the intestine and an adjacent lymph node at autopsy in a transplant recipient who died in the emergency department before workup could be done. Note the bile staining of the raised intestinal mucosal surface of the lesions. A section had already been taken out of the lymph node before the photograph was taken.
Transplant rejection sufficiently severe to cause gross pathology in the first few months following transplantation has become rare. In the following image is a transplanted heart 3 months following transplantation with slight subtle variation in brown coloration and tiny areas of mild congestion, which was all the gross pathology manifested by moderate rejection. If early (acute) rejection is more severe, it can be manifested by pallor, mottling, or hemorrhage.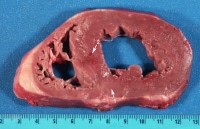 ...">
...">Slight subtle variation in brown coloration (arrow 1) and tiny areas of mild congestion (arrow 2) representing heart transplant rejection.
Transplant rejection can take the form of infarction when vascular rejection leads to thrombosis. Most transplanted organs encountered at autopsy have been in the recipient for years, and many of these have suffered rejection over those years that could be called chronic. Such rejection is generally manifested by scarring. Scarring caused by chronic rejection in transplanted lungs takes the form of obliterative bronchiolitis, which is generally not evident grossly by itself, but it is commonly associated with other conditions, such as pneumonia and diffuse alveolar damage. The cut surface of a transplanted lung with a combination of old and active obliterative bronchiolitis, exudative phase diffuse alveolar damage, and subacute pneumonia is shown below.
Transplanted lung with a combination of old and active obliterative bronchiolitis, exudative phase diffuse alveolar damage, and subacute pneumonia (particularly in the mid left aspect of the picture).
Kidney transplants that are lost to T-cell-mediated rejection focused on the tubulointerstitial compartment in the acute phase, days to weeks following transplantation, sometimes called "white graft rejection," are pale yellow-brown and enlarged, with less pallor in the medulla, especially along the corticomedullary junction.16 In contrast, kidney transplants lost to vascular rejection tend to be mottled red and blue, and to have infarcts, which may be hemorrhagic.
Chronically rejected kidney transplants are usually small and scarred; they may have mottled red-brown areas or small hemorrhagic infarcts.16 Renal allografts scarred from chronic rejection, hypertension-induced nephrosclerosis, calcineurin inhibitor toxicity, preexisting donor disease, or any combination of these conditions have a nonspecific appearance previously diagnosed as "chronic allograft nephropathy," but this term was dropped in the last revision of the renal rejection classification scheme.17
Bacterial infections in transplant patients generally have the same gross pathology as in patients without transplants. The image below demonstrates areas of tan consolidation representing subacute pneumonia due toKlebsiella and Enterobacter in a transplanted lung. Fungal and mycobacterial infections commonly produce nodular lesions, most notably in the lungs.18 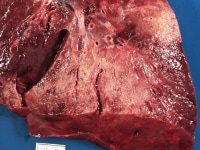
Areas of tan consolidation representing subacute pneumonia due to Klebsiella andEnterobacter.
Special Dissections
In addition to standard dissection, optimum autopsies of transplant recipients require special dissections. Solid organ transplants have surgical anastomoses subject to disease and these should be investigated. All the anastomoses of a transplanted organ should be dissected. This is generally easiest if the autopsy is done using the en masse ("Rokitansky") method of removing all the organs in a single block of tissue. If the prosector wants to use the individual organ (Virchow) technique, it will be necessary to dissect the anastomoses before removing the transplanted organ. The kidney is a special case, because the native kidneys are usually left in place and the transplanted kidney is usually in the pelvis. In fact, an inadequately prepared or careless prosector can destroy the renal allograft anastomoses in the process of evisceration and/or fail to retrieve the kidney transplant.
Kidney transplants have arterial, venous, and ureteral anastomoses. Iliac arteries and veins are usually anastomosed to the transplant blood vessels, and the ureter is implanted into the bladder. There may be stenosis at any of these anastomoses. Ureterovesical anastomotic stenosis can be associated with dilatation of the ureter and renal pelvis.
Liver transplants have hepatic arterial, portal venous, vena caval, and biliary anastomoses. Disease is most likely in the hepatic arterial anastomosis. Stenosis is perhaps the most common hepatic arterial anastomotic problem, but infection, rejection, or lymphoproliferative disorder can all be found there. Finding the hepatic arterial anastomosis can be a challenge, especially if it has been a long time since transplantation; opening the celiac trunk until reaching this anastomosis is usually the best approach, but fibrous adhesions can make this extremely difficult.
The second most problematic liver transplant anastomosis is the biliary. The biliary anastomosis can be within the common bile duct or it can be to a loop of jejunum brought up to the liver in a roux-en-Y fashion. Stenosis and infection are the most common diseases at the biliary anastomosis. Infection is more likely with a jejunobiliary anastomosis, because the absence of a valve can allow intestinal flora bacteria into the biliary tree in combination with immunosuppression impairing the host defense against them.
Lung transplants have arterial, venous, and bronchial anastomoses. The vascular anastomoses of lung transplants rarely have gross pathology, but the bronchial anastomoses are a frequent site of disease, particularly infection, which can produce inflammation, ulceration, and even perforation.
Heart transplants have aortic, pulmonary arterial, and bilateral atrial anastomoses, but these rarely have gross pathology.
Intestinal transplants have arterial, venous, and proximal and distal intestinal anastomoses; these are not especially likely to have gross pathology.
Pancreas transplants are done with a segment of duodenum and have arterial, venous, and intestinal anastomoses. The vascular anastomoses are usually to iliac blood vessels, and the duodenal portion of the transplant may be connected to the small intestine or to the bladder. Pancreatitis can erode these anastomoses.
Multivisceral transplants of intestine plus liver and pancreas have aortic, vena caval, and proximal and distal intestinal anastomoses. These anastomoses are of large caliber and rarely demonstrate macroscopic disease.
The arterial connections of some transplanted abdominal organs, especially liver, sometimes have a segment of another donor artery used to extend the hepatic or other artery. This means there are 2 anastomoses, proximal and distal, and the one most likely diseased is the distal anastomosis. As a general principle, the smaller the structures anastomosed, the more likely the anastomosis has a problem (and the more difficult it is to dissect).Special Autopsy Procedures
Protocols for the microscopic sections for transplanted organs at autopsy are available. The protocols at the University of Pittsburgh Medical Center are as follows:Microscopic Examination and Findings
The microscopic diagnosis of transplant rejection is perhaps best made by pathologists who routinely sign out specimens from transplant patients, if this is an option. The classification and terminology of rejection is increasingly complex and divergent for different types of allografts, but some generalities are worth noting.
Cell-mediated rejection in all types of allografts is characterized by an infiltrate of lymphocytes, sometimes with an activated or immunoblastic appearance and sometimes accompanied by macrophages, eosinophils, plasma cells, and neutrophils. Below is an example of moderate rejection of a transplanted heart with an infiltrate composed of lymphocytes and a few macrophages associated with degeneration of the myocyte in the center. Cell-mediated rejection is frequently focused on blood vessels, airways, bile ducts or renal tubules.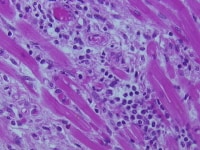
Moderate rejection with an infiltrate of lymphocytes and few macrophages associated with degeneration of the myocyte in the center.
The following image illustrates obliterative bronchiolitis, also called bronchiolitis obliterans, which is the histologic definition of chronic lung transplant rejection.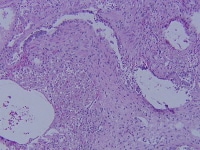
Obliterative bronchiolitis, the histologic definition of chronic lung transplant rejection.
Humoral rejection features the accumulation of fibrinoid material, platelets, and neutrophils, particularly in small blood vessels. The use of immunostaining for C4d has allowed for better diagnosis of humoral rejection.20Immunostaining for C4d in small blood vessels, especially capillaries, is helpful in evaluating transplanted kidneys for rejection and is positive in approximately 35% of affected patients.16 It is important to note, however, that positive immunostaining for C4d must be combined with the presence of circulating anti-donor antibodies in order to diagnose antibody-mediated kidney transplant rejection.
Immunostaining for C4d is also helpful for diagnosing antibody-mediated rejection of heart transplants, but it must be combined with histologic features of antibody-mediated rejection (capillary endothelial swelling or denudation and macrophages in capillaries) in order to make a diagnosis of antibody-mediated heart transplant rejection.21There is also a high rate of background staining, and standardization of interpretation is not complete.22
The diagnosis of antibody-mediated lung transplant rejection is difficult to make, at least in part because capillary injury is a feature of nonspecific diffuse alveolar damage (acute lung injury), various infections, and cellular rejection.23 Criteria for the diagnosis have not been worked out, and immunostaining for C4d has a low sensitivity and specificity for the detection of possible antibody-mediated rejection.24 Transplanted livers are much less susceptible to antibody-mediated rejection perhaps partly because Kupffer cells phagocytose the antibodies or immune complexes with them.11
More severe rejection is associated with evidence of tissue injury. In the acute phase, this injury must be differentiated from ischemic/reperfusion injury by the simultaneous presence of evidence of rejection, but the evidence of injury should be of comparable severity with the evidence of rejection to attribute the injury to the rejection, because patients can have both. Transplant rejection is becoming less common and less severe as more effective immunosuppressive therapies become available. The advent of tacrolimus, mycophenolate, sirolimus, and alemtuzumab (anti-CD52 antibody) has significantly decreased the incidence and severity of rejection. If present, cellular rejection must be differentiated from posttransplant lymphoproliferative disorder. The majority of lymphoproliferative lesions are positive for Epstein-Barr virus by immunostaining or in situ hybridization, which can commonly facilitate this differentiation.25
Transplant recipients can die of all sorts of infections due to bacteria, fungi, mycobacteria, and viruses. Bacteria, particularly multidrug resistant hospital flora bacteria such as Acinetobacter, Pseudomonas, and Enterococcus, but also Staphylococcus aureus associated with catheter-related infection, are perhaps the most common cause of infections in transplant patients. The incidence of various opportunistic infections complicating transplantation is continuously evolving with changes in immunosuppressive therapy and especially with changes in the prophylactic antibiotics given to transplant recipients. Zygomycosis, fungal infection with Mucor, Rhizopus, and related species, for instance, seems to be becoming more common as newer antifungal prophylaxis makes old scourges such as candidiasis and aspergillosis uncommon but fail to prevent zygomycosis.26 The use of antibiotics has also selected out resistant organisms and allowed, for example, aresurgence of cytomegalovirus infections due to ganciclovir-resistant strains.27
Immunostains are widely available for some of the more common pathogens in transplant recipients, including cytomegalovirus, herpes simplex virus, varicella-zoster virus, adenovirus, Toxoplasma, and Pneumocystis, but "special stains" for microorganisms (Gram, Grocott, and acid-fast) remain helpful as well for identifying opportunistic infections.Ancillary and Adjunctive Studies
Blood for aerobic and anaerobic cultures, a piece of lung tissue for aerobic culture, and a piece of spleen for aerobic culture are routinely taken at some autopsy centers and are high yield in autopsies of transplant recipients.28 Lung transplant patients are especially prone to lung infections and these include anaerobic, mycobacterial, fungal and viral infections, so that lung cultures for these types of pathogens are frequently worthwhile in the autopsy of a lung transplant recipient.
As a general principle, nodular lesions in transplant recipients are more likely to be fungal (or mycobacterial) infections than in nontransplant patients. Also as a general principle, if a lesion is worth culturing for fungus, it is worth culturing for mycobacteria, and vice versa.28 The very name "mycobacteria" means funguslike bacteria, because the clinical syndromes and pathology of mycobacterial infections are so similar to fungal infections. Both mycobacteria and fungi tend to produce necrotizing granulomatous infections, which may grossly resemble cheese.
It is a common misassumption that meaningful cultures cannot be obtained if a pus pocket, granuloma, or abscess is encountered in the course of doing an autopsy, but the true pathogen will be present in far greater concentration than contaminants in the culture of an abscess, and granulomas are most likely to be due to fungi or mycobacteria. The culture media for these types of organisms have antibiotics in them to eliminate contaminant organisms.28Multimedia
 upper right, a..." style="border-top-width: 1px; border-right-width: 1px; border-bottom-width: 1px; border-left-width: 1px; border-top-style: solid; border-right-style: solid; border-bottom-style: solid; border-left-style: solid; border-top-color: rgb(0, 102, 153); border-right-color: rgb(0, 102, 153); border-bottom-color: rgb(0, 102, 153); border-left-color: rgb(0, 102, 153); ">
upper right, a..." style="border-top-width: 1px; border-right-width: 1px; border-bottom-width: 1px; border-left-width: 1px; border-top-style: solid; border-right-style: solid; border-bottom-style: solid; border-left-style: solid; border-top-color: rgb(0, 102, 153); border-right-color: rgb(0, 102, 153); border-bottom-color: rgb(0, 102, 153); border-left-color: rgb(0, 102, 153); ">Media file 1: Fistula from the iliac artery (upper right, above probe) to the jejunum (lower left, immediately above the forceps) at the site of a pancreas transplant. 
Media file 2: Posttransplant lymphoproliferative disorder in the intestine and an adjacent lymph node at autopsy in a transplant recipient who died in the emergency department before workup could be done. Note the bile staining of the raised intestinal mucosal surface of the lesions. A section had already been taken out of the lymph node before the photograph was taken.  ..." style="border-top-width: 1px; border-right-width: 1px; border-bottom-width: 1px; border-left-width: 1px; border-top-style: solid; border-right-style: solid; border-bottom-style: solid; border-left-style: solid; border-top-color: rgb(0, 102, 153); border-right-color: rgb(0, 102, 153); border-bottom-color: rgb(0, 102, 153); border-left-color: rgb(0, 102, 153); ">
..." style="border-top-width: 1px; border-right-width: 1px; border-bottom-width: 1px; border-left-width: 1px; border-top-style: solid; border-right-style: solid; border-bottom-style: solid; border-left-style: solid; border-top-color: rgb(0, 102, 153); border-right-color: rgb(0, 102, 153); border-bottom-color: rgb(0, 102, 153); border-left-color: rgb(0, 102, 153); ">Media file 3: Slight subtle variation in brown coloration (arrow 1) and tiny areas of mild congestion (arrow 2) representing heart transplant rejection. 
Media file 4: Transplanted lung with a combination of old and active obliterative bronchiolitis, exudative phase diffuse alveolar damage, and subacute pneumonia (particularly in the mid left aspect of the picture). 
Media file 5: Areas of tan consolidation representing subacute pneumonia due to Klebsiella and Enterobacter. 
Media file 6: Moderate rejection with an infiltrate of lymphocytes and few macrophages associated with degeneration of the myocyte in the center. 
Media file 7: Obliterative bronchiolitis, the histologic definition of chronic lung transplant rejection.
Sunday, October 17, 2010
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
(Enlarge Image)
Subscribe to:
Post Comments (Atom)
0 comments:
Post a Comment